5 Ways a Laboratory Information System Revolutionizes Lab Efficiency
Imagine walking into a bustling laboratory where every second counts and the pressure to deliver precise results is palpable. In this fast-paced healthcare and research landscape, laboratories are not just places of analysis; they are critical hubs for innovation and patient care. Enter the Laboratory Information System (LIS) — a game-changing tool that transforms how labs operate. By streamlining workflows, reducing errors, and enhancing productivity, an LIS empowers laboratories to meet the ever-growing demands of their field. This article explores five key ways a Laboratory Information System can revolutionize lab efficiency, from automating processes to ensuring regulatory compliance.
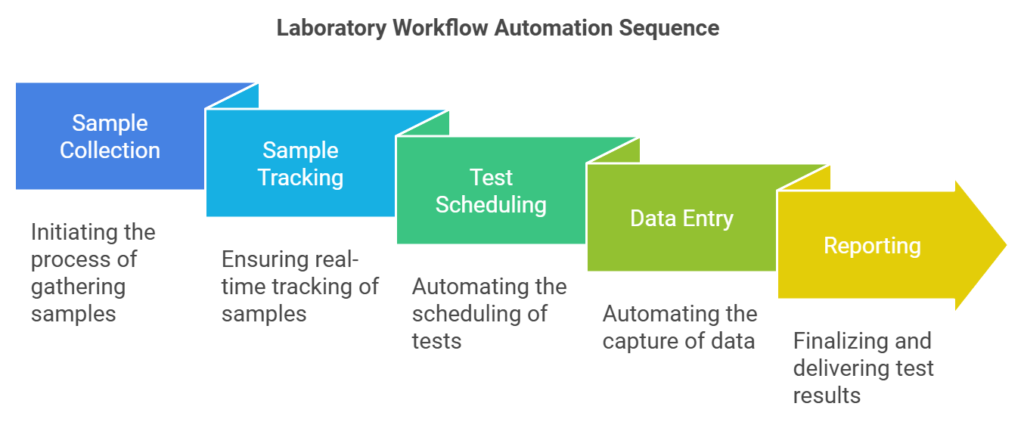
1. Streamlining Workflow Automation
One of the most significant contributions of a Laboratory Information System is its ability to automate workflows. Labs traditionally rely on manual processes, which are prone to human error and inefficiencies. An LIS eliminates these issues by automating routine tasks such as:
- Sample Tracking: From collection to reporting, an LIS ensures that every sample is tracked in real-time, reducing the risk of misplacement or duplication.
- Test Scheduling: Automated scheduling ensures optimal resource utilization while minimizing downtime.
- Data Entry: Manual data entry is replaced by automatic data capture, reducing transcription errors and saving time.
By automating these processes, labs can allocate their resources more effectively. For example, technicians can focus on high-value tasks such as analysis and interpretation rather than spending hours on administrative duties. Workflow automation also ensures that laboratories can handle higher volumes of samples without delays, a critical factor in environments such as clinical diagnostics and pharmaceutical research.
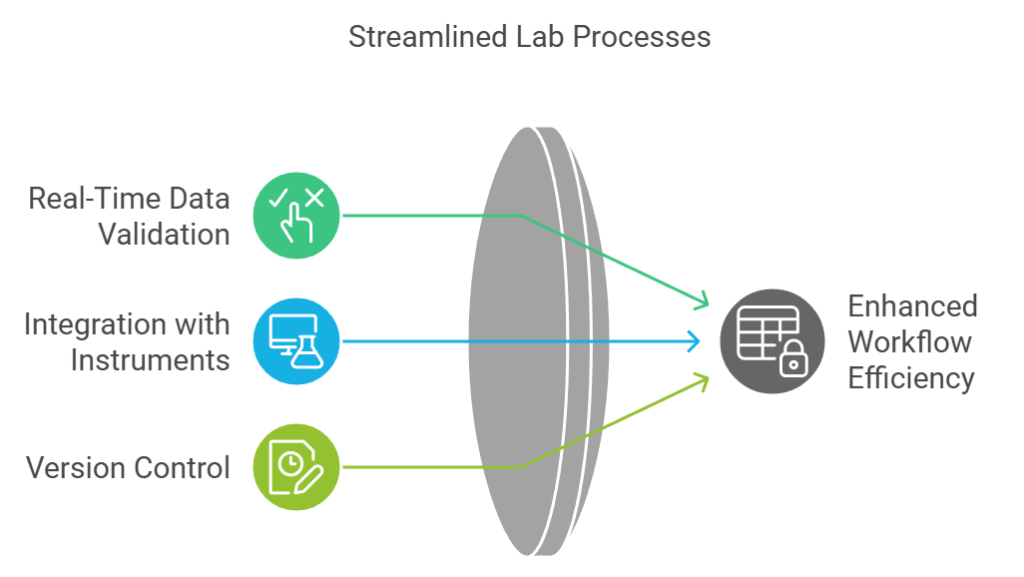
2. Enhancing Data Accuracy and Integrity
Maintaining accurate and reliable data is a cornerstone of any laboratory operation. An LIS enhances data integrity by centralizing information and implementing standardized protocols across all stages of testing. Key features include:
- Real-Time Data Validation: The system cross-checks inputs to flag inconsistencies or errors immediately.
- Integration with Instruments: An LIS directly connects with lab instruments, ensuring seamless data transfer and eliminating manual transcription errors.
- Version Control: All changes to data are tracked, providing a clear audit trail for regulatory compliance.
With these capabilities, laboratories can minimize errors that could compromise results. This not only improves the reliability of test outcomes but also builds trust with clients and stakeholders, a critical aspect of maintaining a lab’s reputation.
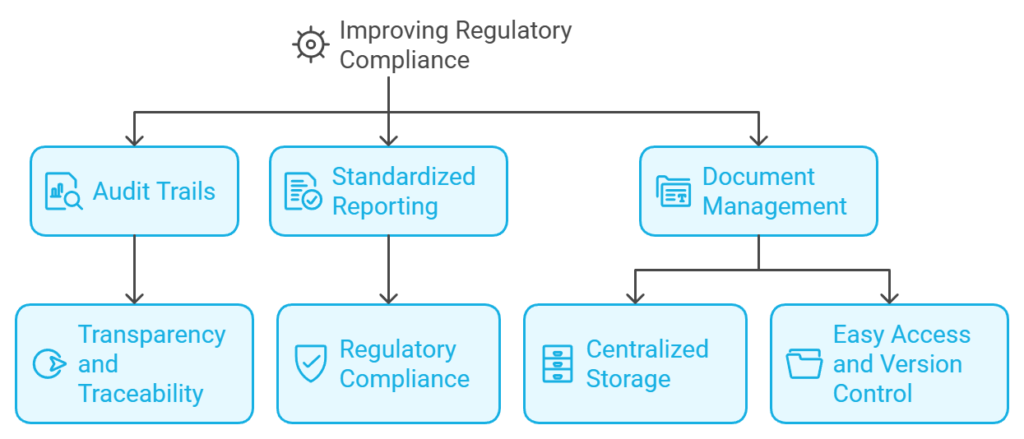
3. Improving Regulatory Compliance
Compliance with industry standards and regulations such as CLIA, CAP, and ISO 15189 is a critical challenge for laboratories. A Laboratory Information System simplifies compliance by embedding regulatory requirements into its workflows. Some of the ways an LIS ensures compliance include:
- Audit Trails: Comprehensive logs of all lab activities, from sample handling to result reporting, provide transparency and traceability.
- Standardized Reporting: Automatically generated reports adhere to regulatory formats, reducing the risk of non-compliance.
- Document Management: Centralized storage of protocols, SOPs (Standard Operating Procedures), and other documentation ensures easy access and version control.
By automating compliance-related tasks, labs reduce the likelihood of penalties or certification issues. Additionally, the time saved can be redirected toward innovation and value-added activities, further enhancing lab efficiency.
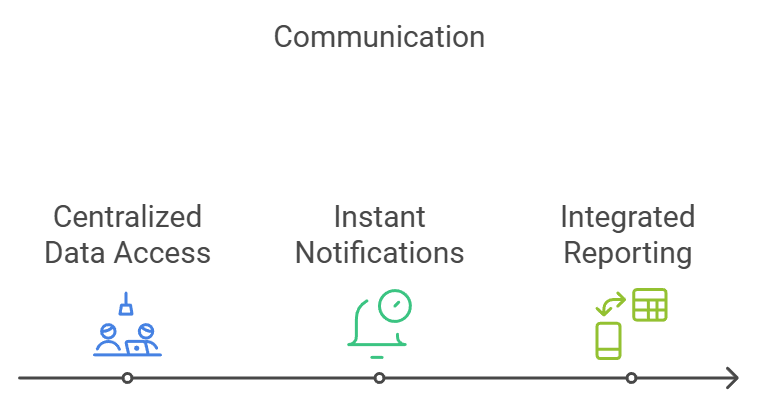
4. Facilitating Seamless Communication and Collaboration
Modern laboratories often involve multi-disciplinary teams working across different locations. An LIS fosters better communication and collaboration by serving as a central hub for all lab data and processes. Key benefits include:
- Centralized Data Access: All team members can access up-to-date information from a single platform, reducing the need for back-and-forth communication.
- Instant Notifications: Automated alerts inform relevant personnel about sample status, test results, or system issues, ensuring timely action.
- Integrated Reporting: Results can be shared instantly with clinicians, researchers, or clients, speeding up decision-making.
This enhanced connectivity not only improves internal collaboration but also strengthens relationships with external stakeholders. For example, in a clinical setting, faster communication of test results can directly impact patient outcomes.
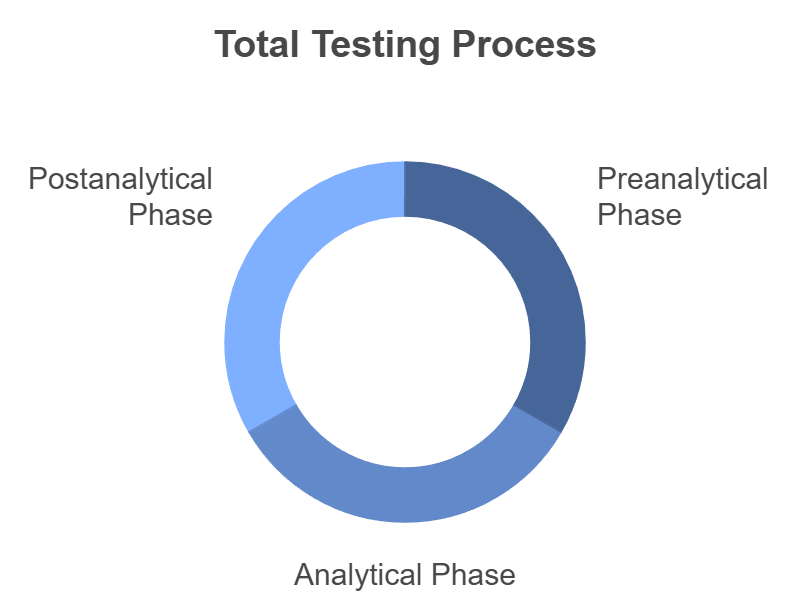
5. Optimizing Resource Management Through the Total Testing Process
Effective resource management is essential for maintaining lab efficiency across all phases of the total testing process: preanalytical, analytical, and postanalytical. A Laboratory Information System optimizes each phase through automation:
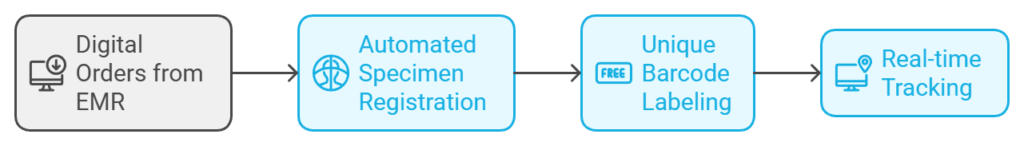
- Preanalytical Phase:
- Specimen Registration (Accessioning): An LIS automates specimen registration by accepting digital orders directly from Electronic Medical Records (EMR), minimizing manual entry errors.
- Tracking with Barcode Labels: Each specimen is assigned a unique barcode label for real-time tracking throughout the testing process, ensuring accurate identification and reducing mislabeling risks.
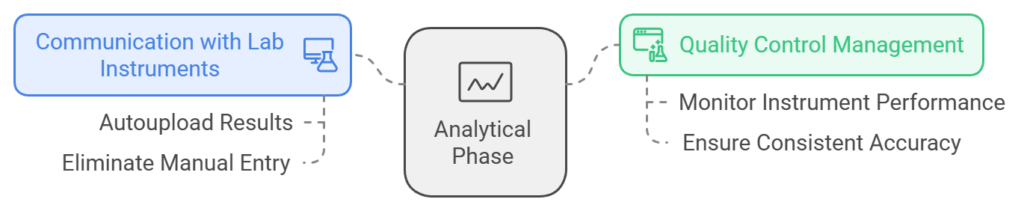
- Analytical Phase:
- Communication with Lab Instruments: An LIS integrates seamlessly with laboratory instruments to autoupload results directly into the system, eliminating manual result entry.
- Quality Control Management: Automated QC management tools monitor instrument performance in real-time, ensuring consistent accuracy in test results.
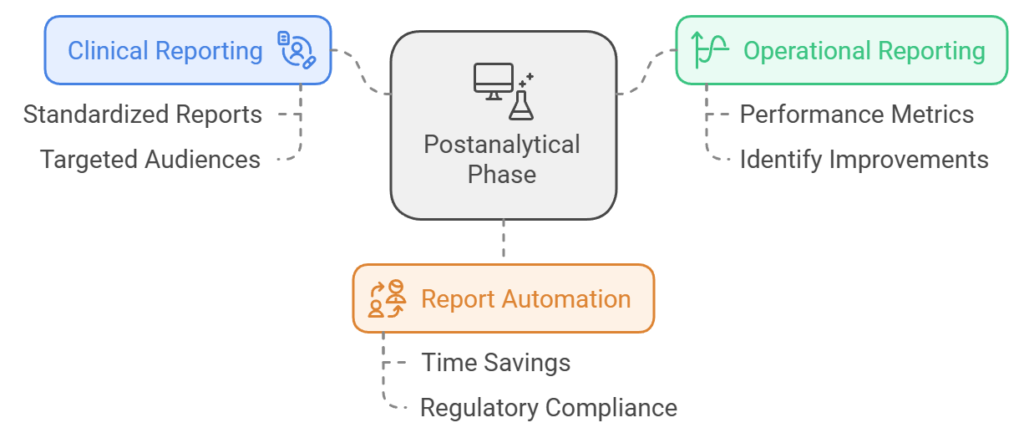
- Postanalytical Phase:
- Clinical Reporting: An LIS automates clinical reporting by generating standardized reports tailored to specific audiences such as clinicians or patients.
- Operational Reporting: The system provides insights into lab performance metrics through automated operational reports that help identify areas for improvement.
- Report Automation: Automated report generation saves time while ensuring compliance with regulatory standards.
By optimizing these three phases through automation, an LIS not only enhances resource management but also improves overall laboratory efficiency. This comprehensive approach allows labs to operate more effectively while maintaining high-quality standards.
A Laboratory Information System is no longer a luxury but a necessity for modern laboratories striving for efficiency, accuracy, and compliance. By automating workflows, ensuring data integrity, simplifying compliance, enhancing collaboration, and optimizing resource management, an LIS transforms how labs operate. Whether in clinical, research, or industrial settings, adopting an LIS is a strategic investment that delivers measurable benefits in both the short and long term.
Laboratories that implement an LIS not only improve their operational efficiency but also position themselves as leaders in their respective fields. By embracing this technology, labs can meet the growing demands of their industries while maintaining the highest standards of quality and reliability.